أكسيد المنغنيز الثلاثي
| |
| الأسماء | |
|---|---|
| أسماء أخرى
dimanganese trioxide, manganese sesquioxide, manganic oxide, manganous oxide
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.878 |
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | Mn2O3 |
| كتلة مولية | 157.8743 g/mol |
| المظهر | brown or black crystalline |
| الكثافة | 4.50 g/cm3 |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | 0.00504 g/100 mL (alpha form) 0.01065 g/100 mL (beta form) |
| قابلية الذوبان | insoluble in ethanol, acetone soluble in acid, ammonium chloride |
| القابلية المغناطيسية | +14,100·10−6 cm3/mol |
| البنية | |
| البنية البلورية | Bixbyite, cI80 |
| الزمرة الفراغية | Ia3 (No. 206) |
| ثابت العقد | a = 942 pm |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−971 kJ·mol−1[1] |
| Standard molar entropy S |
110 J·mol−1·K−1[1] |
| المخاطر | |
| NFPA 704 (معيـَّن النار) | |
| مركبات ذا علاقة | |
أنيونات أخرى
|
manganese trifluoride, manganese(III) acetate |
كاتيونات أخرى
|
chromium(III) oxide, iron(III) oxide |
مركـّبات ذات علاقة
|
manganese(II) oxide, manganese dioxide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Manganese(III) oxide is a chemical compound with the formula Mn2O3. It occurs in nature as the mineral bixbyite (recently changed to bixbyite-(Mn)[2][3]) and is used in the production of ferrites and thermistors.
Preparation and chemistry
Heating MnO2 in air at below 800 °C produces α-Mn2O3 (higher temperatures produce Mn3O4).[4] γ-Mn2O3 can be produced by oxidation followed by dehydration of manganese(II) hydroxide.[4] Many preparations of nano-crystalline Mn2O3 have been reported, for example syntheses involving oxidation of MnII salts or reduction of MnO2.[5][6][7]
Manganese(III) oxide is formed by the redox reaction in an alkaline cell:
- 2 MnO2 + Zn → Mn2O3 + ZnO[بحاجة لمصدر]
Manganese(III) oxide Mn2O3 must not be confused with MnOOH manganese(III) oxyhydroxide. Contrary to Mn2O3, MnOOH is a compound that decomposes at about 300 °C to form MnO2.[8]
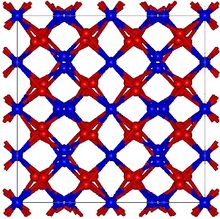
Structure
Mn2O3 is unlike many other transition metal oxides in that it does not adopt the corundum (Al2O3) structure.[4] Two forms are generally recognized, α-Mn2O3 and γ-Mn2O3,[9] although a high pressure form with the CaIrO3 structure has been reported too.[10]
α-Mn2O3 has the cubic bixbyite structure, which is an example of a C-type rare earth sesquioxide (Pearson symbol cI80, space group Ia3, #206). The bixbyite structure has been found to be stabilised by the presence of small amounts of Fe3+, pure Mn2O3 has an orthorhombic structure (Pearson symbol oP24, space group Pbca, #61).[11] α-Mn2O3 undergoes antiferromagnetic transition at 80 K. [12]
γ-Mn2O3 has a structure related to the spinel structure of Mn3O4 where the oxide ions are cubic close packed. This is similar to the relationship between γ-Fe2O3 and Fe3O4.[9] γ-Mn2O3 is ferrimagnetic with a Néel temperature of 39 K.[13]
ε-Mn2O3 takes on a rhombohedral ilmenite structure (the first binary compound known to do so), wherein the manganese cations divided equally into oxidation states 2+ and 4+. ε-Mn2O3 is antiferromagnetic with a Néel temperature of 210 K.[14]
References
- ^ أ ب Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Bixbyite-(Mn)".
- ^ IMA 21-H: Redefinition of bixbyite and definition of bixbyite-(Fe) and bixbyite-(Mn). CNMNC Newsletter, 64, 2021; Mineralogical Magazine, 85, 2021).
- ^ أ ب ت Greenwood, N. N. (1997). Chemistry of the Elements (2nd Edition ed.). Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Shuijin Lei; Kaibin Tang; Zhen Fang; Qiangchun Liu; Huagui Zheng (2006). "Preparation of α-Mn2O3 and MnO from thermal decomposition of MnCO3 and control of morphology". Materials Letters. 60: 53. doi:10.1016/j.matlet.2005.07.070.
- ^ Zhong-Yong Yuan; Tie-Zhen Ren; Gaohui Du; Bao-Lian Su (2004). "A facile preparation of single-crystalline α-Mn2O3 nanorods by ammonia-hydrothermal treatment of MnO2". Chemical Physics Letters. 389 (1–3): 83. doi:10.1016/j.cplett.2004.03.064.
- ^ Navin Chandra; Sanjeev Bhasin; Meenakshi Sharma; Deepti Pal (2007). "A room temperature process for making Mn2O3 nano-particles and γ-MnOOH nano-rods". Materials Letters. 61 (17): 3728. doi:10.1016/j.matlet.2006.12.024.
- ^ Thomas Kohler; Thomas Armbruster; Eugen Libowitzky (1997). "Hydrogen Bonding and Jahn-Teller Distortion in Groutite,α-MnOOH, and Manganite,γ-MnOOH, and Their Relations to the Manganese Dioxides Ramsdellite and Pyrolusite". Journal of Solid State Chemistry. 133 (2): 486–500. doi:10.1006/jssc.1997.7516.
- ^ أ ب Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ High Pressure Phase transition in Mn2O3 to the CaIrO3-type Phase Santillan, J.; Shim, S. American Geophysical Union, Fall Meeting 2005, abstract #MR23B-0050
- ^ Geller S. (1971). "Structure of α-Mn2O3, (Mn0.983Fe0.017)2O3 and (Mn0.37Fe0.63)2O3 and relation to magnetic ordering". Acta Crystallogr B. 27 (4): 821. doi:10.1107/S0567740871002966.
- ^ Geller S. (1970). "Magnetic and Crystallographic Transitions in Sc+, Cr+, and Ga+ Substituted Mn2O3". Physical Review B. 1 (9): 3763. doi:10.1103/physrevb.1.3763.
- ^ Kim S. H; Choi B. J; Lee G.H.; Oh S. J.; Kim B.; Choi H. C.; Park J; Chang Y. (2005). "Ferrimagnetism in γ-Manganese Sesquioxide (γ−Mn2O3) Nanoparticles". Journal of the Korean Physical Society. 46 (4): 941.
- ^ Ovsyannikov, Sergey V.; Tsirlin, Alexander A.; Korobeynikov, Igor V.; Morozova, Natalia V.; Aslandukova, Alena A.; Steinle-Neumann, Gerd; Chariton, Stella; Khandarkhaeva, Saiana; Glazyrin, Konstantin; Wilhelm, Fabrice; Rogalev, Andrei; Dubrovinsky, Leonid (2021-09-06). "Synthesis of Ilmenite-type ε-Mn 2 O 3 and Its Properties". Inorganic Chemistry (in الإنجليزية). 60 (17): 13348–13358. doi:10.1021/acs.inorgchem.1c01666. ISSN 0020-1669. PMID 34415155. S2CID 237242460.
- CS1 errors: unsupported parameter
- CS1 errors: extra text: edition
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- Articles with unsourced statements from August 2015
- Manganese(III) minerals
- Sesquioxides
- أكاسيد معدنية إنتقالية
